Goal: When you have finished this laboratory exercise you will understand
- how foods are frozen
and you will learn
- how temperature inside a food varies during freezing
- different types of freezing systems used in the industry
- how freezing time is measured
- the effect of air velocity and product size on the freezing time
-
Industrial freezers may be broadly classified as direct contact and non-contact types. The following animations show the difference between the two types:
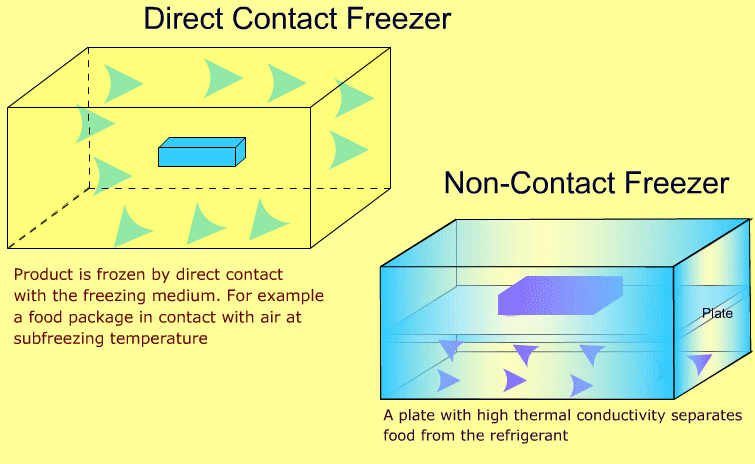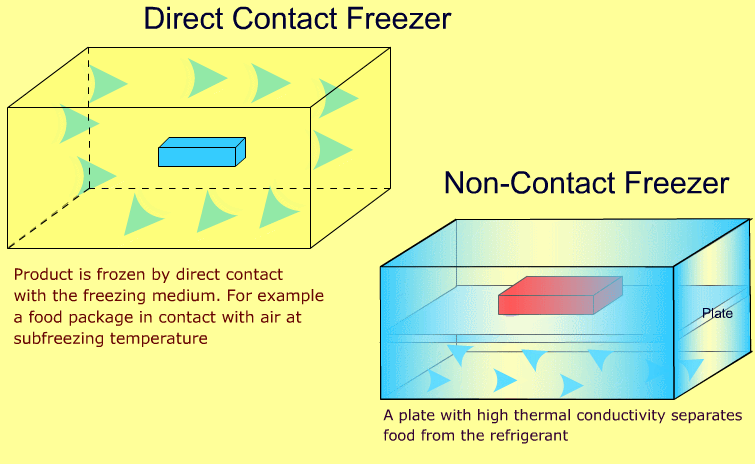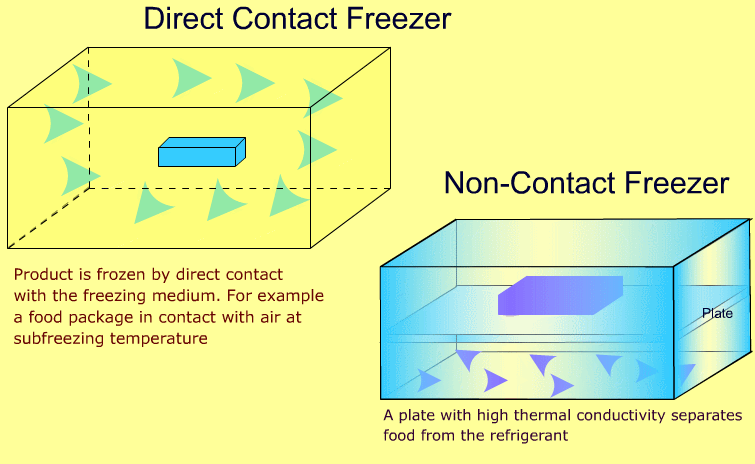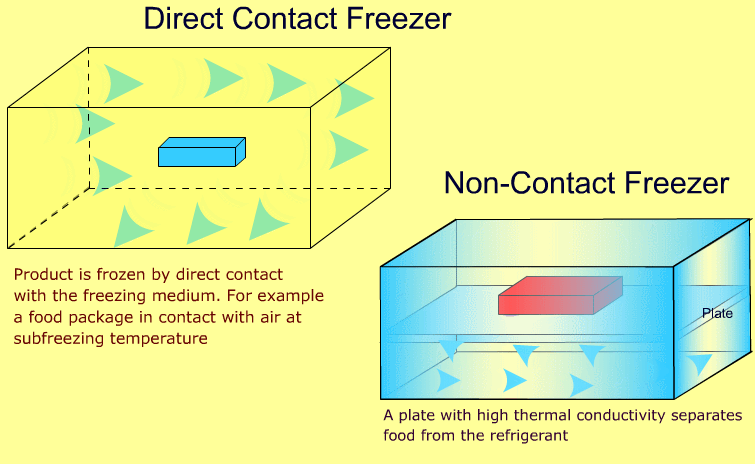
-
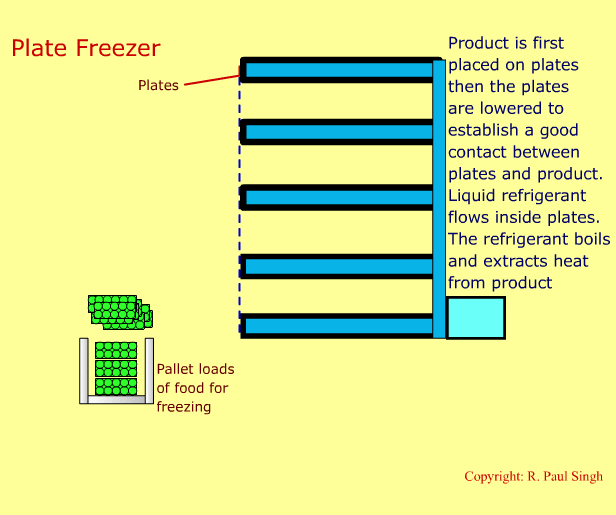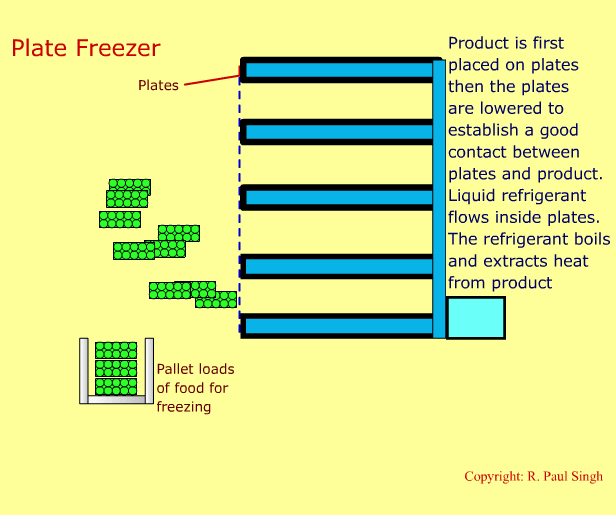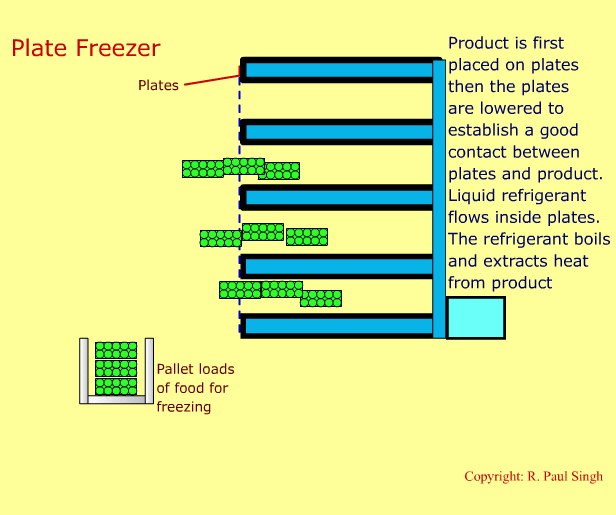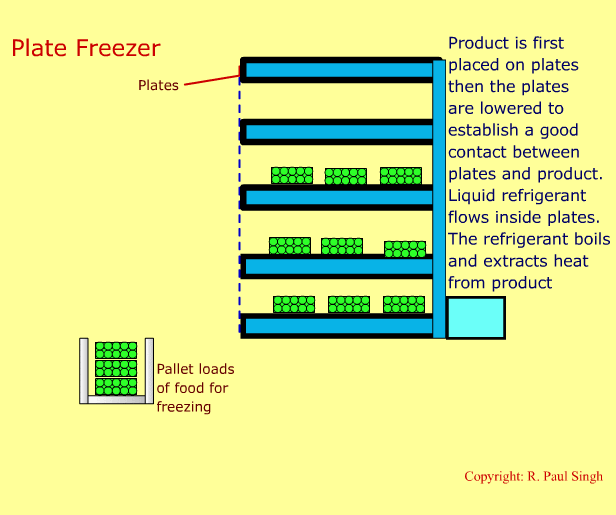
Plate Freezing Systems
These systems involve first placing food in a brick-shaped package. The package is then placed between metal plates. Pressure is applied on the plates and the package. A refrigerant boils inside the hollow space in the plates. Thus, heat transfers from the food into the refrigerant. The next slide shows an animation of a plate freezer. -
-
In a fluidized freezer, product is lifted off the belt by air at subfreezing temperature.
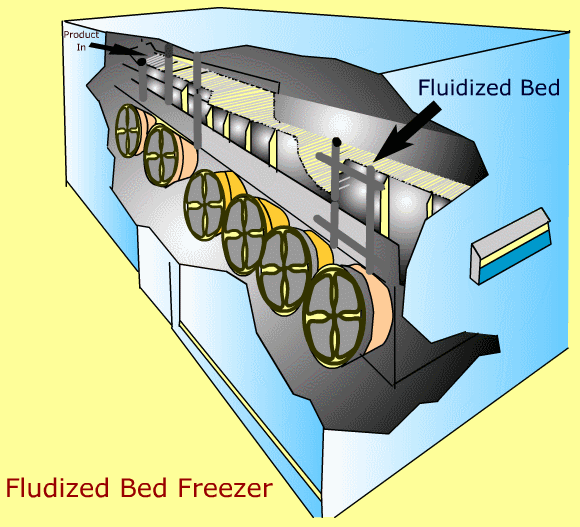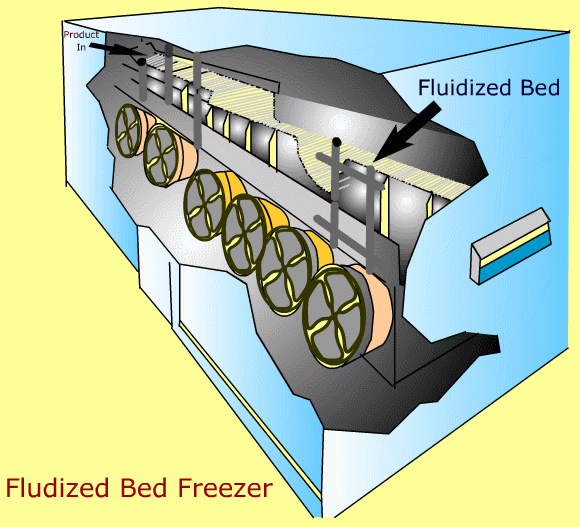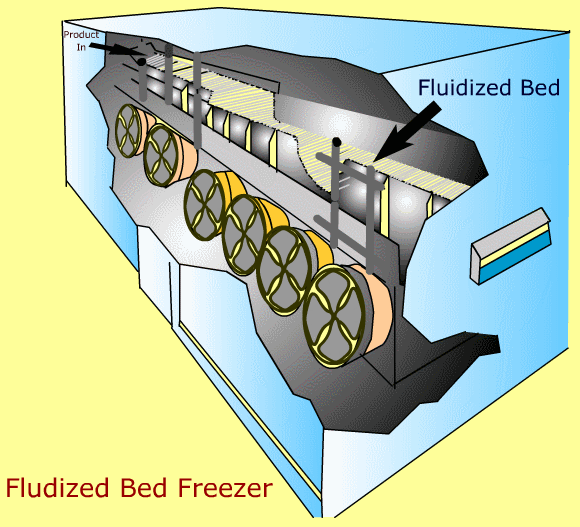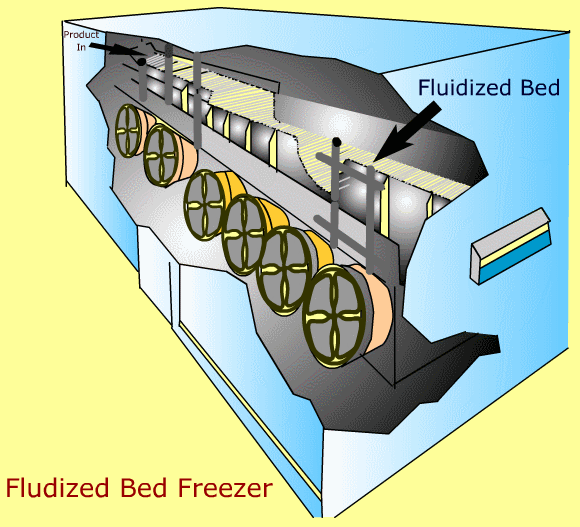
-
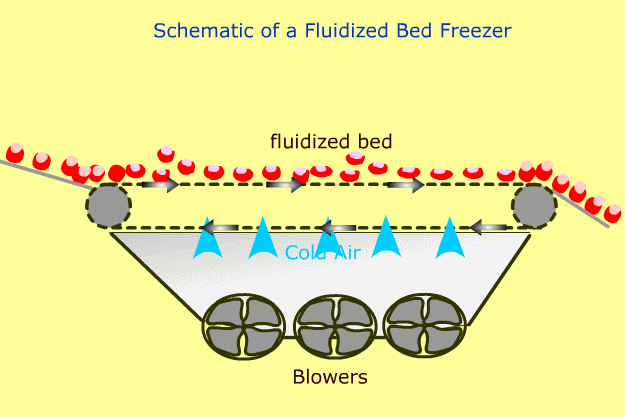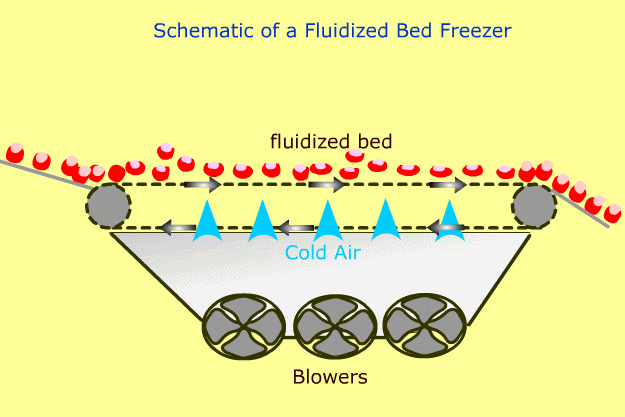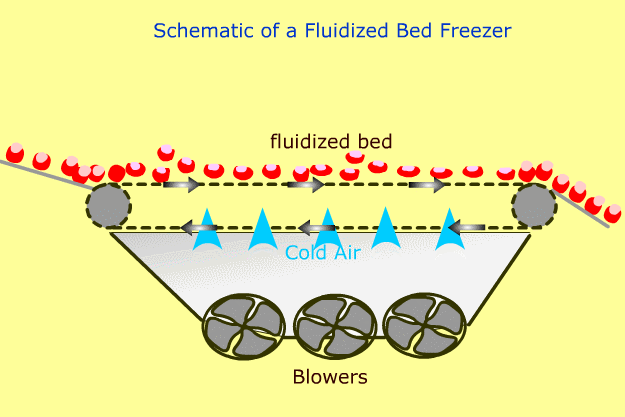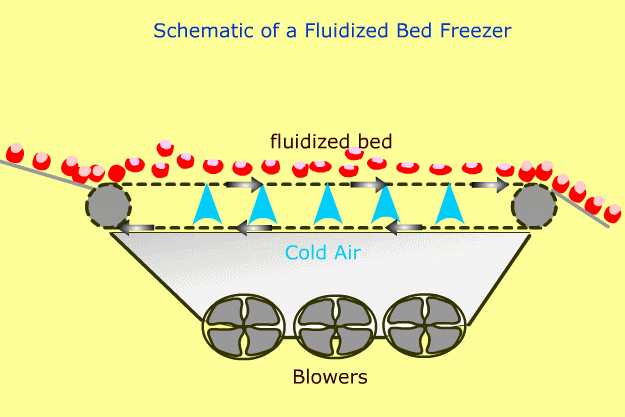
-
Cryogenic Freezing Systems
In these freezers, food is either immersed in a liquid cryogen (e.g. liquid Nitrogen) or the cryogen is sprayed directly on the food.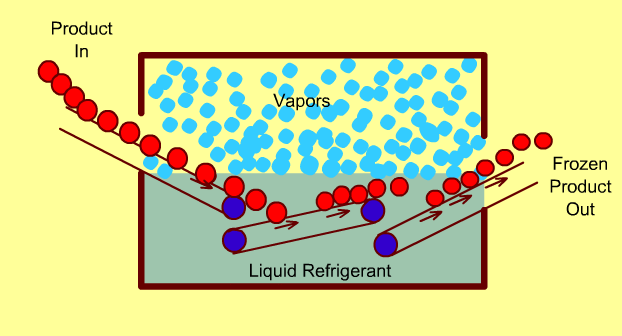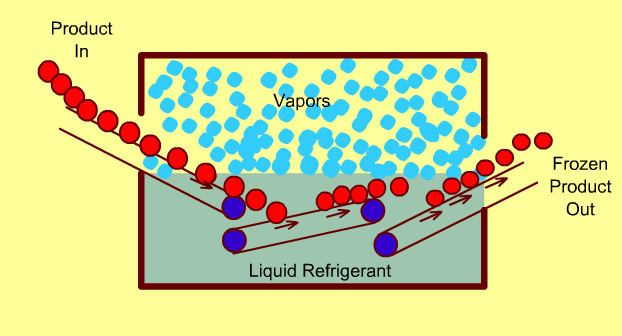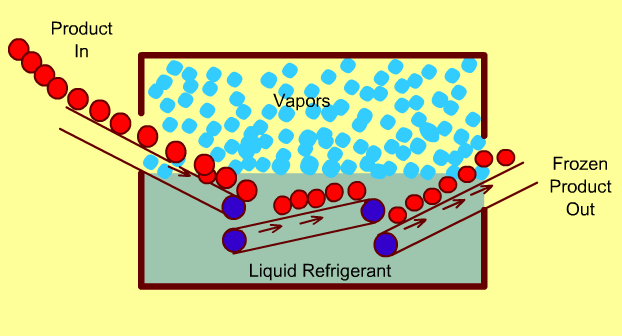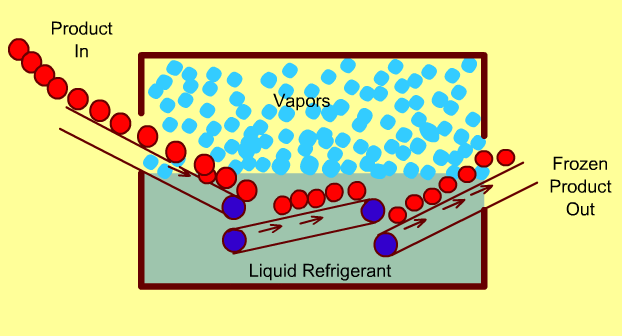
-
Spiral Freezer
In a spiral freezer, product is conveyed on a spiral belt.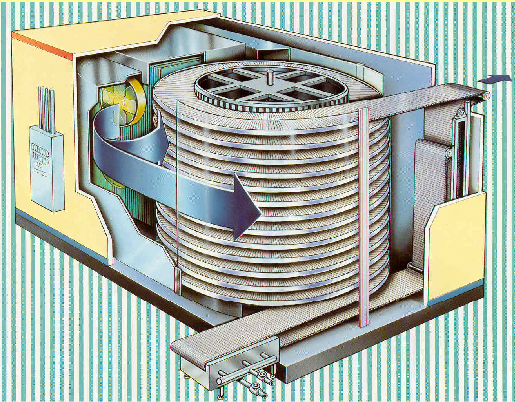
-
Air Impingement Freezer
In an impingement freezer, high velocity jets are used to freeze foods.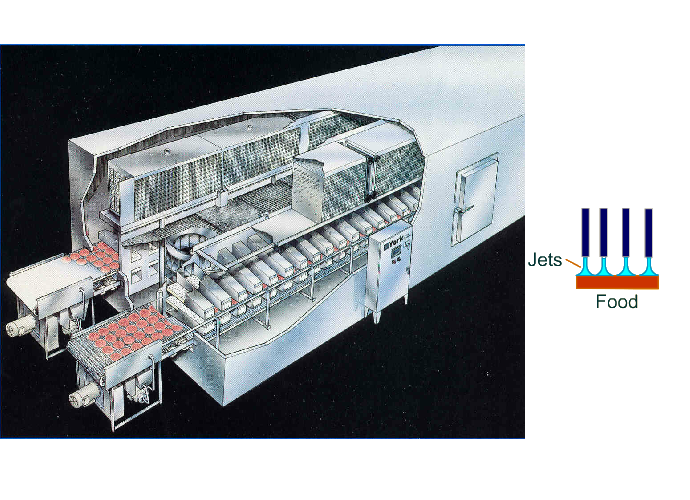
-
In a laboratory experiment, following procedures are used to measure freezing time.
1) Using cork-borers of different diameters, prepare cylindrical samples of potato for freezing.
-
2) Insert a thermocouple lengthwise into the sample, paying extra attention that the measuring junction is located at the thermal center of the cylinder.
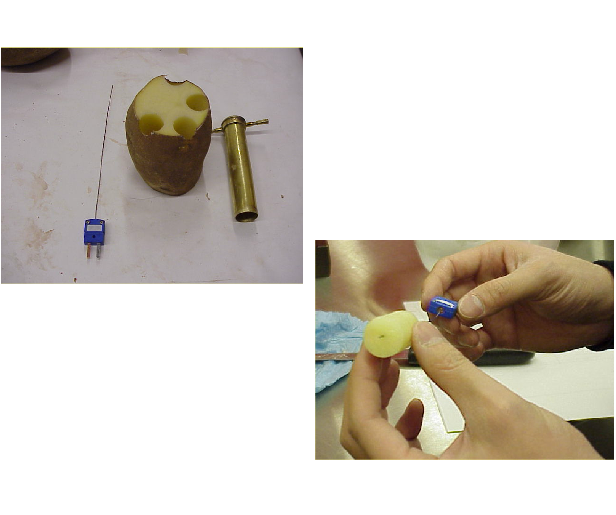
-
3) Place potato cylinder with thermocouple in an air-blast freezer and record temperature while it is frozen.

-
4) After the temperature at the center of the sample drops to -20oC or lower, remove the sample from freezer and let it thaw. Remove the thermocouple and cut the sample along the radial direction to confirm that the thermocouple was located at the center of the potato sample, if not then repeat the trial.

-
In a virtual experiment, you have a choice of either freezing water or a potato. For either of these items, the shape has been pre-selected as a short cylinder (e.g. water in a cylindrical container). Enter the dimensions of the cylinder, diameter and length. Then select initial temperature, final center temperature, air temperature (air is used as a freezing medium) and air velocity. The results will include freezing time and a plot of temperature vs time for the freeing medium (air) and the product center temperature. The temperature vs time data are presented in a spreadsheet.
Note: when freezing water, you should use an initial temperature close to 0oC to avoid the effect of thermal convective currents.
-
Freezing process involves a change in phase as water converts into ice crystals. This process involves unsteady state heat transfer, with a change in thermal and physical properties. Perhaps the most important aspect of the mathematical description of freezing is the changing physical and thermal properties. The change in properties as a function of temperature is often modeled using appropriate mathematical functions. A typical freezing curve is shown as follows. Note that during most of the freezing time, latent heat must be removed to accomplish change of phase.
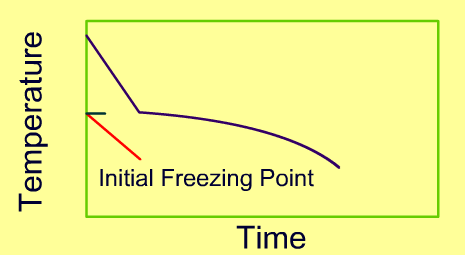
-
This experiment involved obtaining freezing time to allow the center temperature to reach -18 or -20oC. Additionally, temperature-time data were obtained for each trial. From the data saved on the spreadsheet, use the following steps to analyze the data:
1) Plot the temperature vs. time data for freezing the given sample, water or potato, for similar operating conditions of product diameter, air temperature and air velocity. Identify regions of the plot that represent removal of sensible and latent heat. Observe any differences in plots for potato and water. -
2) Using the plots of temperature history for each condition, compare each plot and observe the influence of product size and air velocity.
- Discuss how the center temperature changes with time for samples of water and potato. Comment on the shape of the plot.
- Discuss the effect of air velocity on the freezing time.
- How does air velocity influence the freezing process?
- Use an analytical procedure for calculating freezing time (such as Planck's or Pham's method) and compare the results with those you obtained from the experiment.
- Barbosa-Canovas, G.V., Ma, L. and Braletta, B. (1997). "Food Processing Laboratory Manual," Technomic Publishing Co., Inc. Lancaster.
- Fellows, P.J. (1997). "Food Processing Technology, Principles and Practices'" Woodhead Publishing Series, Cambridge.
- Singh, R.P., and Heldman, D.R.(2009). "Introduction to Food Engineering," 4th ed., Academic Press, London.